Cooking Ice Cream with Liquid Nitrogen
What is Liquid Nitrogen?
 If you think back to grade school, you learned that there are several states of matter, depending on the material and temperature. The three main ones that we encounter in our daily lives are solid, liquid, and gas. You know that ice, steam, and water are all the same thing, just at different temperatures. You are probably also aware that metal and plastic will melt if they get too hot. We can think of these materials as "frozen" at room temperature.
If you think back to grade school, you learned that there are several states of matter, depending on the material and temperature. The three main ones that we encounter in our daily lives are solid, liquid, and gas. You know that ice, steam, and water are all the same thing, just at different temperatures. You are probably also aware that metal and plastic will melt if they get too hot. We can think of these materials as "frozen" at room temperature.
Water, as you know, freezes at 0° Celsius (32° Fahrenheit), and it becomes a gas (steam) when it boils at 100° Celsius (212° Fahrenheit).
At normal temperatures on Earth, Nitrogen is a gas. It's actually all around you. About 78% of the air you are breathing right now is made of nitrogen — only 21% is oxygen, and the rest is other chemicals like carbon dioxide, argon, etc.
Have you ever seen water forming on the outside of a glass of a cold beverage? Your glass is not leaking; the cold glass is cooling water vapor in the air until it becomes a liquid again. In the same way, air can be cooled low enough to make the nitrogen form a liquid. The difference is that nitrogen's boiling point is very low, so we have to cool the nitrogen to a very low temperature to convert it to a liquid. That temperature is -196° Celsius (-340° Fahrenheit).
Water freezes at 0 and boils at 100, so liquid nitrogen is essentially "two boilings below freezing." That's pretty cold! As you know, our bodies need to stay within certain ranges. If they get too hot, they suffer heat burns. If they get too cold, they suffer cold burns like frostbite. Obviously, touching boiling water is painful and can quickly lead to serious injury. In the same way, contact with liquid nitrogen can be painful and dangerous.
Since we don't ordinarily experience anything this cold, working with liquid nitrogen can seem almost magical.
Nitrogen atoms typically pair up into an N2 molecule, just like oxygen. For this reason, liquid nitrogen is commonly called LN2.
Many dangerous chemicals have nitrogen in them, such as ammonium nitrate used in Space Shuttle booster fuel or nitroglycerin, the key explosive component in dynamite. For this reason, many non-chemists immediately associate LN2 with being incredibly dangerous. While Many safety precautions employed to mitigate the dangers of cold, LN2 is not an explosive.
Contrary to popular belief, liquid nitrogen will not make everything shatter. Many materials normally flexible will shatter when they are very cold, and smashing rubber balls is a popular science demonstration.
Making ultra-cold foods
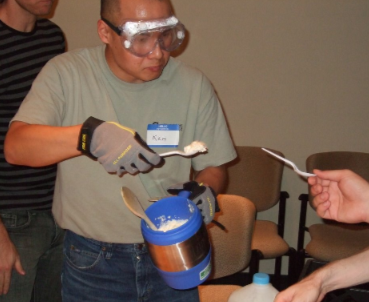 Ice cream is, by far, the most popular food to make with liquid nitrogen. Basically, you prepare the ice cream mix with whatever recipe you usually use. Pour some into a large bowl and slowly pour LN2 in while quickly mixing. To make a smooth ice cream, you need to keep the ice cream mix moving, otherwise, it will just freeze into ice blobs. I suggest doing several small batches so you can practice getting the timing just right.
Ice cream is, by far, the most popular food to make with liquid nitrogen. Basically, you prepare the ice cream mix with whatever recipe you usually use. Pour some into a large bowl and slowly pour LN2 in while quickly mixing. To make a smooth ice cream, you need to keep the ice cream mix moving, otherwise, it will just freeze into ice blobs. I suggest doing several small batches so you can practice getting the timing just right.
We've done the ice cream as a large demonstration with 50-400 people watching. In the future, I'd like to be much more intimate and allow people to mix their own ice cream in an insulated coffee mug. Get the ice cream moving like you were mixing chocolate milk, then pour in the LN2.
You can also go the other way. The ice cream product "Dippin' Dots" is made by slowly dripping the ice cream mix into a vat of LN2, then fishing the ice cream drops out or draining the LN2. Make sure you allow them to warm up to a safe eating temperature before serving. Regular ice cream comes with a built-in safety indicator — if it is completely solid and can't be scooped, it is too cold.
I've experimented with a lot of foods. Low-density foods are best. Whipped creams, marshmallows, doughnuts, and cheese puffs all have interesting effects. Whipped cream and marshmallows shatter when you bite them. Porous bread products can produce water vapor smoke as you eat them.
Higher-density foods, on the other hand, are downright dangerous. Cheese and Jello™, for example, will instantly stick to your tongue and start burning it. The first time this happened, I saved my tongue by jumping under the emergency shower. Now I keep a sports bottle of water handy. This limitation really cuts down on the number of foods you can dunk in the LN2 and enjoy, though I am experimenting with LN2 as a food preparation technology.
I typically use high-density plastic dishware. Dog bowls are ideal because they do not rest on the table and therefore don't waste as much LN2. Their shape also reduces the chances of tipping, an added safety feature.
LN2 Safety
Liquid nitrogen has many non-obvious, counter-intuitive properties that can make it hazardous to use. This is not an exhaustive discussion of all possible hazards. You should research the topic and/or work with an experienced person if you decide to start working with liquid nitrogen.LN2 is, of course, radically cold. You should wear eye protection at all times, and face shields are recommended. Wearing clothing that completely covers your skin and does not quickly absorb liquids is also recommended. Ensure that LN2 will not become trapped in your clothing in pockets, pant cuffs, etc. Be able to quickly remove protective clothing in the event it comes in contact with LN2.
Most materials look exactly the same when they are cold, making it easy to accidentally touch or grab a dangerously cold object. Because the moisture in your skin can quickly freeze, you may become stuck to the object and unable to release it, causing further unwanted contact. For this reason, you should wear thermally insulated gloves. Special cryo-gloves are the best protection, although they are not designed to withstand continued submersion. It is a good idea to keep a ready supply of warm water to reheat frozen body parts and separate them from cryogenic materials. Yes, this is like calling the fire department when a kid licks a wintertime flagpole.
LN2 is cold enough to cause oxygen in the air to liquefy, causing liquid oxygen to form on top of the LN2. Concentrated oxygen can cause combustion to rapidly and dangerously accelerate, as demonstrated by George Goble, who pours LOX on his barbecue to instantly prepare for grilling — with a giant fireball.
Like water expanding to form steam, nitrogen will expand greatly as it boils and may displace oxygen in the air. Because no carbon dioxide is produced, your lungs cannot sense the lack of oxygen, and you may suffocate. Ensure proper ventilation when using, storing or transporting LN2 in confined spaces, especially automobiles and elevators.
The boiling point of liquids also changes with altitude and air pressure. For this reason, use caution when transporting LN2 by aircraft or elevator. The use of a freight elevator or unmanned passenger elevator cab is highly recommended when transporting significant quantities of LN2.
LN2 will rapidly boil and expand when heated. Use only special LN2-approved funnels. Likewise, do not submerge tubes in LN2 — the warm tube will cause LN2 to rapidly expand inside the tube and create a geyser. The best way to determine the amount of LN2 remaining in your dewar is to weigh it.
This rapid warming also causes the Leidenfrost Effect. If you were to drip some LN2 onto your skin, it would boil and float on the gas produced. This allows you to touch the LN2. You can reach into a bowl and quickly splash it. Be warned, however, that your skin will quickly cool to the point that the liquid no longer floats and burns will occur. This effect has led to some to speculate that the safest way to handle LN2 is to handle it naked.
Do not store LN2 in a sealed container. It is always boiling and will eventually rupture the container, leading to an explosion.
How I got started
I first got interested in liquid nitrogen after hearing a presentation on molecular gastronomy. Chef-scientists use all kinds of weird industrial and laboratory techniques to make unique foods. Around the same time, I read the article in Popular Science about people who were making Liquid Nitrogen Ice Cream.One day I suggested to a friend of mine that this would be a fun thing to do at a science fiction convention. She was able to find a dewar, purchase the nitrogen and make ice cream — a huge success. I suggested it would be fun to try for my Independence Day party, and she helped us make it. The leftover nitrogen stayed with me and I had a blast the rest of the weekend trying classical LN2 experiments.
Eventually, I purchased my own handling equipment and started working with LN2 on my own.
Obtaining liquid nitrogen, and other practical aspects.
The first step is to obtain a safe way to store LN2. The best way is with a vacuum dewar, essentially a large Thermos bottle. In addition to being super-insulated, the lid is designed to allow nitrogen gas to safely vent as it slowly boils.The dewar I have stores 10 liters of LN2. It takes 30-45 days for that quantity to completely boil off, depending on air pressure and temperature. The dewar cost about $600. Smaller ones, about the size of a beer pitcher, are in the $200-300 range. Thermos bottles and beer coolers do not have adequate insulation to store liquid nitrogen. They can also spontaneously crack and leak nitrogen. While they can be useful for temporary handling, I don't think any dealer will sell it to you that way.
I obtain LN2 from industrial gas suppliers like AirGas and Praxair. These companies supply many kinds of gases for welding, medical, etc. The cost is about $4.00 per liter.
You will typically need to have an account with the company before you can purchase from them, and this may take 30 days to establish. They don't usually sell to individuals, so if you have a business you will be able to expedite the process. Some companies will dispense it on the spot, while others will only deliver it. Some will want you to leave your dewar overnight.
When I first started, I used shop gloves. They are better than nothing and give you about 10 seconds of holding something cold before you really don't want to be holding it anymore. Be aware that they are not liquid-proof and that LN2 can seep in along the stitching... but if you don't have $300 for real cryo-gloves, they are better than nothing. Rubber and latex gloves offer almost no protection.
I transport the filled dewar in the back seat of my car. I wedge it in place to keep it from tipping. I don't carry enough to require the hazardous materials placards, though I have some small ones that I've made and sometimes use.

